Oxidation Number of Hydrogen
In KH the oxidation number of hydrogen is -1 and the oxidation number of K is 1. When H is directly attached to strongly electropositive metals such as K the H atom is present in the form.
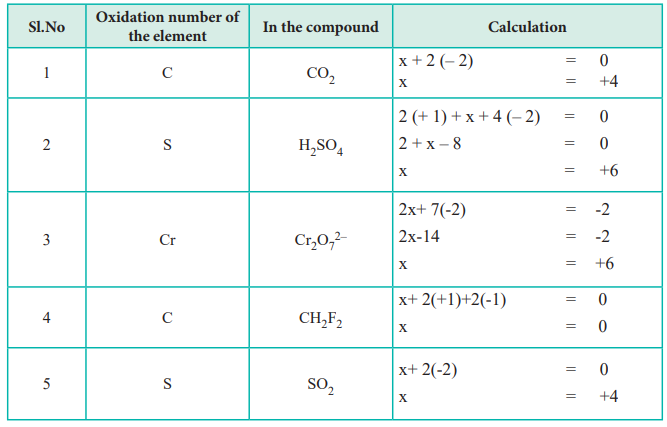
Rules For Oxidation Numbers Study Guide Inspirit
The oxidation number of hydrogen is 1 except when it is bonded to metals in binary compounds that is compounds containing two elements.
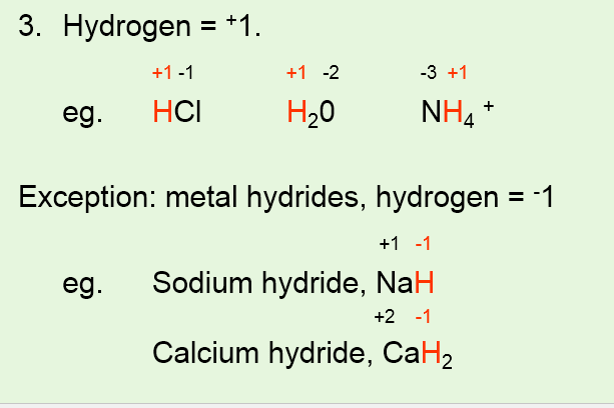
. This is formally the charge left on the atom of interest when the bond that joins it to another atom is BROKEN with the charge ie. The oxidation number is frequently used interchangeably with an Oxidation state. Hydrogen has an oxidation number of 1 in most compounds.
The major exception is when hydrogen is combined with metals as in N a H ce NaH NaH or L i A l H X 4. The oxidation number of a monatomic ion equals the. The oxidation number of a free element is always 0.
In a neutral compound all Oxidation Numbers must add up to zero. It does not have an overall charge and is a neutral molecule. Hydrogen has an oxidation number of 1 in water because it has lost one electron.
The hydrogen atom H exhibits an oxidation state of 1. One carbon atom is at -3 oxidation state and other carbon atom is at -1 oxidation state. When carbon form compounds with non-metal.
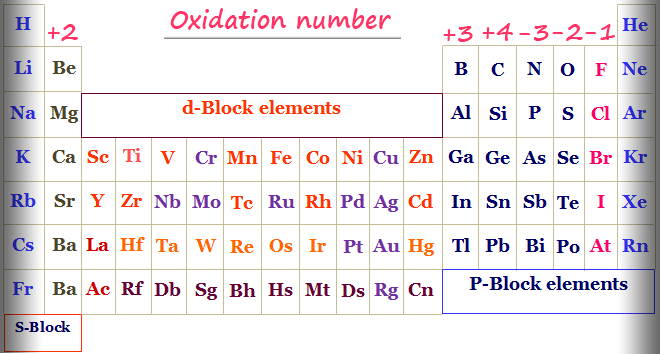
The oxidation number of a free element is always 0. Group 1 1 Group 2 2 Hydrogen with Non-Metals 1 Hydrogen with Metals or Boron -1 Fluorine. For free elements the oxidation number is zero.
In borane even though B is a non-metal each hydrogen displays an oxidation state of -1 again electronegativity of H is 21 as opposed 20 of B Hope this makes sense. Oxidation number of oxygen in oxide ion O 2- is -2 and in peroxide ion O-O 2- is -1. For the compound sodium hydride hydrogen is bonded to sodium which is a metal so the oxidation number of hydrogen is -1.
The oxidation number of a monatomic ion equals the charge of the ion. Hydrogen has an oxidation number of 1 when combined with non-metals but it has an oxidation number of -1 when combined with metals. Ethanol CH3CH2OH lewis structure should be drawn to find oxidation numbers of elements.
Well how do we assign oxidation numbers. Carbon atoms oxidation number is 2Hydrogens oxidation number is 1Oxygens oxidation number is -2. NaH and CaH 2 are some examples.
Oxygen has three possible oxidation. 6 The oxidation number of hydrogen is 1 when it is combined with more electronegative elements most nonmetals and 1 when it is combined with more electropositive elements. Oxidation number of hydrogen in proton H is 1 and in hydride is -1.
The usual oxidation number of hydrogen is 1. When hydrogen forms compounds with metals hydrogens oxidation number is -1. The algebraic sum of the oxidation numbers of.
For example in LiH and NaH its oxidation. However when the Hydrogen is bonded to a metal LiH or NaH for example then the charge is 1-. Because the single oxygen atom has received a total of two electrons one from each hydrogen oxygen has.
The Oxidation state of Hydrogen is 1 when in a regular compound. The atoms in He and N 2 for example have oxidation numbers of 0. It is because the stock notation of oxidation numbers is the basis of the periodic property.
However when bonded with an element with less electronegativity than it it exhibits an oxidation number of -1. H 2 is a free element meaning it is not bound to any atoms other than its own. Answer 1 of 2.
The vast emission of hydrogen chloride HCl in the chlorination industry has raised great interest in producing chlorine Cl 2 directly from HClIn this work thermodynamic.

Oxidation Number State Definition Rules How To Find And Examples
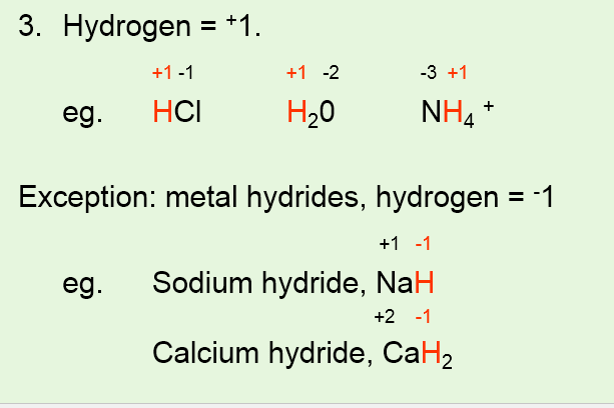
Oxidation Numbers Vce Chemistry
Oxidation Number Periodic Table Elements Definition Rules
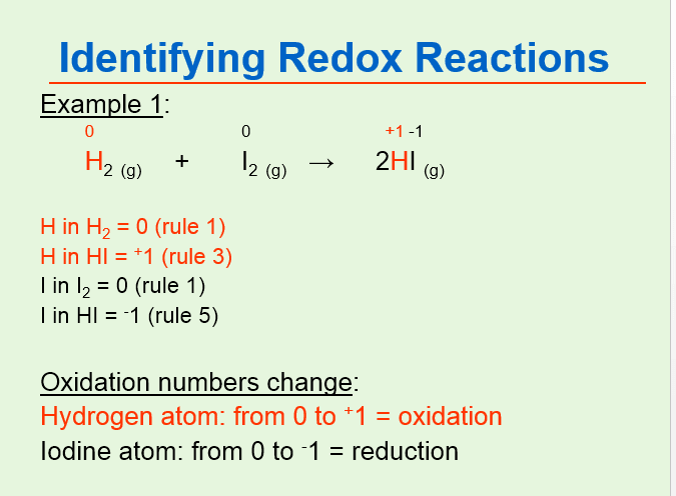
Oxidation Numbers Vce Chemistry
0 Response to "Oxidation Number of Hydrogen"
Post a Comment